Lab 1 Acids And Bases
Brønsted Concept of Acids and Bases
- Page ID
- 1281
In 1923, chemists Johannes Nicolaus Brønsted and Thomas Martin Lowry independently developed definitions of acids and bases based on the compounds' abilities to either donate or accept protons (\(H^+\) ions). In this theory, acids are defined equally proton donors; whereas bases are defined as proton acceptors. A compound that acts as both a Brønsted-Lowry acid and base of operations together is called amphoteric.
The Brønsted-Lowry Theory of Acids and Bases
Brønsted-Lowry theory of acid and bases took the Arrhenius definition one stride further, every bit a substance no longer needed to exist composed of hydrogen (H+) or hydroxide (OH-) ions in guild to be classified as an acid or base. For instance , consider the following chemical equation:
\[ HCl \; (aq) + NH_3 \; (aq) \rightarrow NH_4^+ \; (aq) + Cl^- \; (aq) \]
Here, muriatic acid (HCl) "donates" a proton (H+) to ammonia (NH3) which "accepts" it , forming a positively charged ammonium ion (NH4 +) and a negatively charged chloride ion (Cl-). Therefore, HCl is a Brønsted-Lowry acid (donates a proton) while the ammonia is a Brønsted-Lowry base (accepts a proton). Also, Cl- is called the conjugate base of operations of the acrid HCl and NHfour + is called the conjugate acrid of the base of operations NH3.
- A Brønsted-Lowry acrid is a proton (hydrogen ion) donor.
- A Brønsted-Lowry base of operations is a proton (hydrogen ion) acceptor.
In this theory, an acid is a substance that tin release a proton (similar in the Arrhenius theory) and a base is a substance that tin can accept a proton. A basic common salt, such as Na+F-, generates OH- ions in water past taking protons from water itself (to brand HF):
\[F^-_{(aq)} + H_2O_{(l)} \rightleftharpoons HF_{(aq)} + OH^-\]
When a Brønsted acid dissociates, information technology increases the concentration of hydrogen ions in the solution, \([H^+]\); conversely, Brønsted bases dissociate by taking a proton from the solvent (water) to generate \([OH^-]\).
- Acid dissociation
\[HA_{(aq)} \rightleftharpoons A^-_{(aq)} + H^+_{(aq)}\]
- Acid Ionization Constant:
\[K_a=\dfrac{[A^-][H^+]}{[HA]}\]
- Base dissociation:
\[B_{(aq)} + H_2O_{(l)} \rightleftharpoons HB^+_{(aq)} + OH^-_{(aq)}\]
- Base Ionization Abiding
\[K_b = \dfrac{[HB^+][OH^-]}{[B]}\]
The determination of a substance equally a Brønsted-Lowery acid or base of operations can just be done by observing the reaction. In the case of the HOH it is a base of operations in the start example and an acrid in the 2nd case.
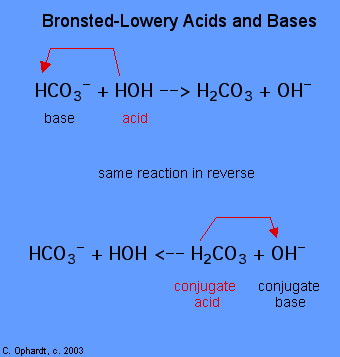
To determine whether a substance is an acid or a base, count the hydrogens on each substance before and afterward the reaction. If the number of hydrogens has decreased that substance is the acid (donates hydrogen ions). If the number of hydrogens has increased that substance is the base (accepts hydrogen ions). These definitions are normally applied to the reactants on the left. If the reaction is viewed in reverse a new acid and base tin can be identified. The substances on the right side of the equation are called conjugate acid and conjugate base compared to those on the left. Also notation that the original acrid turns in the conjugate base of operations later on the reaction is over.
Annotation
Acids are Proton Donors and Bases are Proton Acceptors
For a reaction to be in equilibrium a transfer of electrons needs to occur. The acid will give an electron away and the base volition receive the electron. Acids and Bases that work together in this fashion are called a cohabit pair fabricated upwards of conjugate acids and conjugate bases.
\[ HA + Z \rightleftharpoons A^- + HZ^+ \]
A stands for an Acidic chemical compound and Z stands for a Basic chemical compound
- A Donates H to form HZ+.
- Z Accepts H from A which forms HZ+
- A- becomes conjugate base of HA and in the contrary reaction information technology accepts a H from HZ to recreate HA in order to remain in equilibrium
- HZ+ becomes a cohabit acid of Z and in the reverse reaction information technology donates a H to A- recreating Z in lodge to remain in equilibrium
Questions
- Why is \(HA\) an Acrid?
- Why is \(Z^-\) a Base?
- How tin A- exist a base when HA was and Acid?
- How can HZ+ exist an acid when Z used to be a Base?
- Now that we understand the concept, let's look at an an example with actual compounds! \[ HCl + H_2O \rightleftharpoons H_3O^+ + Cl^¯ \]
- HCL is the acid because it is altruistic a proton to HiiO
- HiiO is the base because H2O is accepting a proton from HCL
- H3O+ is the conjugate acrid because it is altruistic an acid to CL plow into it'southward conjugate acid H2O
- Cl¯ is the conjugate base of operations because it accepts an H from HiiiO to return to it'southward conjugate acid HCl
How can H2O be a base? I thought it was neutral?
Answers
- It has a proton that can be transferred
- It receives a proton from HA
- A- is a cohabit base because it is in need of a H in order to remain in equilibrium and return to HA
- HZ+ is a conjugate acid considering it needs to donate or give away its proton in order to render to it'south previous state of Z
- In the Brønsted-Lowry Theory what makes a compound an element or a base is whether or not information technology donates or accepts protons. If the H2O was in a unlike problem and was instead altruistic an H rather than accepting an H it would be an acid!
Contributors and Attributions
- Sarah Rundle (UCD), Charles Ophardt, Professor Emeritus, Elmhurst College; Virtual Chembook
Lab 1 Acids And Bases,
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Acids_and_Bases/Acid/Bronsted_Concept_of_Acids_and_Bases
Posted by: bryanttretind.blogspot.com


0 Response to "Lab 1 Acids And Bases"
Post a Comment